CDE releases the List of the Second Batch of Overseas New Drugs Urgently Needed in Clinical Settings

On May 29, 2019, NMPA Center for Drug Evaluation issued the Notice on the Issuance of the List of the Second Batch of Overseas New Drugs Urgently Needed in Clinical Settings, which reads as follows:
To implement the policies set forth in the State Council executive meetings and speed up the entry of overseas new drugs urgently needed in clinical practice to China, according to the Announcement on Issues Pertaining to the Review and Approval of Overseas New Drugs Urgently Needed in Clinical Settings (No. 79 of 2018), NMPA and the National Health Commission organized relevant experts to study, demonstrate and select the second batch of overseas new drugs urgently needed in clinical settings, the list of which has been published on the website of CDE. On May 29, 2019, the list of 26 uncontested varieties (such as Biopten Granules) as the second batch of urgently needed overseas new drugs in clinical practice, was officially announced to the public.
The application for marketing of varieties listed in the list of new overseas drugs urgently needed in clinical settings can be submitted directly in accordance with theWork Procedures for Review and Approval of Overseas New Drugs Catering to Clinical Urgent Needs. CDE has established a special channel to speed up the review. For not-yetdeclared product, you can contact us at any time to submit an application for marketing ASAP.
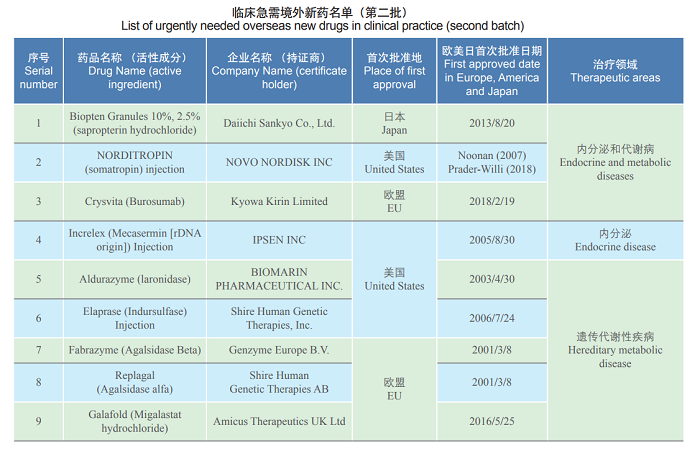
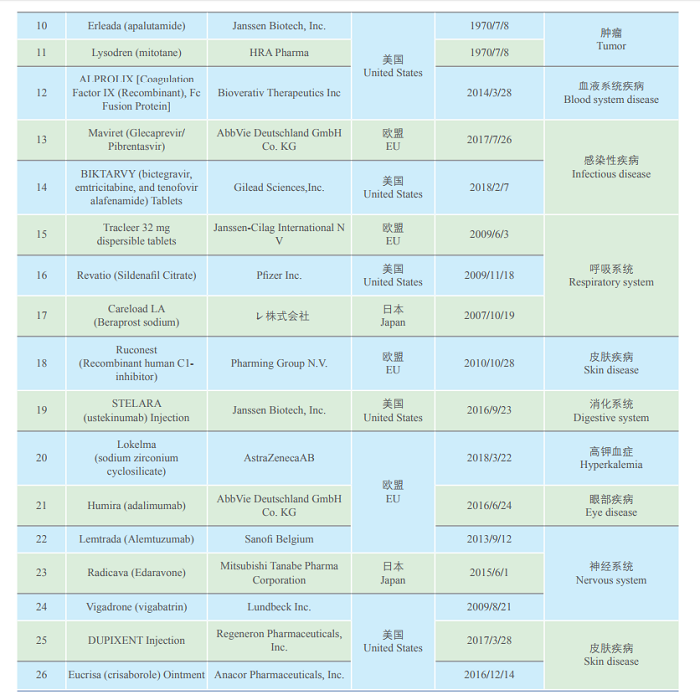
Note: “First approved date in Europe, America and Japan, therapeutic targets, indications, and reasons for listed as clinical imperative” in the original table: Omitted




